Abstract
Background: Despite the use of CD19-directed CAR-T cell therapy (CAR19) for treatment of relapsed/refractory (R/R) B-acute lymphoblastic leukemia (B-ALL), we cannot predict patient response before infusion. While CAR19 therapies show high complete remission (CR) rates, 15% of patients do not respond (NR), while others eventually relapse (REL). The B-ALL tumor microenvironment may impact efficacy of CAR T cell therapy. We evaluated whether pre-infusion bone marrow biopsy (BMB) parameters predict response to CAR-T cell therapy.
Methods: Patients treated with CAR19 for R/R B-ALL from 2012-2021 at the Children's Hospital of Philadelphia were identified. BMBs from pre-CAR and 28 days post-CAR timepoints were evaluated. RNA sequencing-based gene expression profiling was performed (EdgeSeq Whole Transcriptome Panel, HTG Diagnostics). Differential expression (DE) was assessed by DESeq2 in HTG Reveal software. Pathway analysis was done using Metascape. BMBs were evaluated with reticulin special stain and CD3 immunostaining and quantified by expert scoring and digital image analysis. Modified Bauermeister scale ≥ 2 was considered severe fibrosis. Sustained Responders (SR) were defined as patients who did not relapse after achieving CAR19-mediated CR. 63 cases of primary and relapsed B-ALL were stained with CD3 and reticulin to determine baseline variation in fibrosis and CD3 infiltration before any immunotherapy.
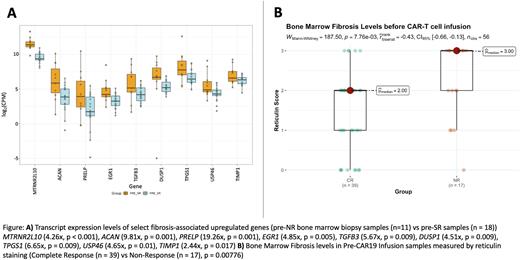
Results: DE and pathway enrichment analysis of NR and SR pre-CAR infusion samples showed significant NR up-regulation of genes in Skeletal System (p < 0.001) and Connective Tissue Development (p < 0.001), Epithelial to Mesenchymal Transition (p < 0.001), and ECM Structural Constituent (p < 0.001) pathways. Cytokine signaling was not significantly different between groups. Several fibrosis-associated genes, TGFB3 (5.67x, p = 0.009), PRELP (19.26x, p = 0.001), EGR1 (4.85x, p = 0.005), and DUSP1 (4.51x, p = 0.009), were among the ten most significantly upregulated genes. NRs also demonstrated the highest levels of pre-CAR fibrosis. 76% of NRs had a fibrosis score ≥ 2 compared to 56.7% for CRs and 46.7% for REL, and a higher average fibrosis score (2.23), compared to CR (1.46), SR (1.59), and REL (1.47) groups (p = 0.00776, p = 0.03, p = 0.00999, respectively; Mann-Whitney U-Test, two-tailed). CD3 staining was inversely correlated to reticulin fibrosis.
Among non-immunotherapy treated patients, 70.3% of primary and 55.6% of relapsed cases had severe fibrosis, while 51.9% of primary and 77.8% of relapsed cases lacked CD3 infiltration. Fibrosis was higher in hyperdiploid, hypodiploid, iAMP21, PAX5-rearranged, and complex genetic subtypes of B-ALL, while CD3 infiltration was lower in PAX5-rearranged, MLL, TCF3-PBX1 and relapsed subtypes of B-ALL.
Conclusions We describe for the first time the association between bone marrow fibrosis and response to CAR19 therapy in B-ALL. ECM and Connective Tissue Development pathway expression in NRs exceeds that of both their treatment-responsive peers, as well as expected physiological expression of bone growth/remodeling. Moreover, severe fibrosis and diminished lymphocytic infiltrates were noted in high-risk genetic subtypes of B-ALL even before administration of any immunotherapy. As such, up-regulated fibrosis-associated genes such as PRELP, EGR1, DUSP1, and TGFB3 could be targeted to improve response to CAR19 therapy. Additionally, reticulin staining is a widely available assay that could be used to assess fibrosis in bone marrow biopsies and serve as a useful heuristic tool in predicting the likelihood of therapy success. Ultimately, evaluation of pre-CAR infusion fibrosis levels in conjunction with other molecular and morphological markers may allow for more informed decision making, targeted therapeutic plan development, and patient outcome optimization in R/R B-ALL.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.